NLO microscopy techniques
Coherent Anti-Stokes Raman Spectroscopy (CARS)
CARS is a type of optical spectroscopy used to study the vibrational modes of molecules through nonlinear interaction of two laser beams. The pump laser excites the molecule, then the probe beam measures the intensity of the Raman-scattered light, which is proportional to the vibrational frequency. This means that it is also a label-free imaging technique that is capable of real-time, nonperturbative examination of living cells and organisms. CARS spectroscopy has many applications in fields such as chemistry, biology, and materials science.
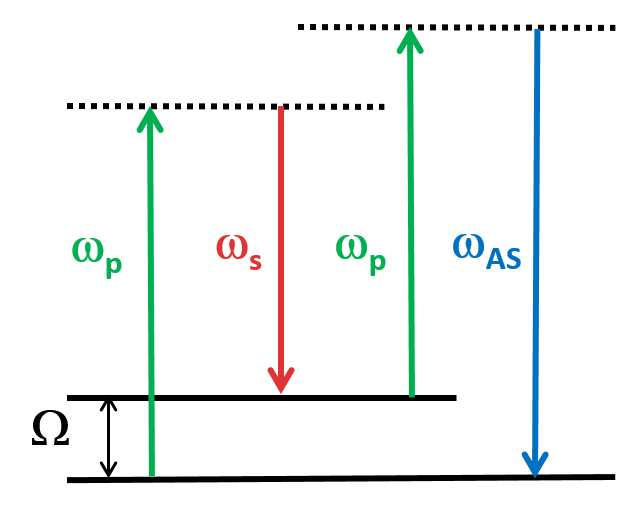
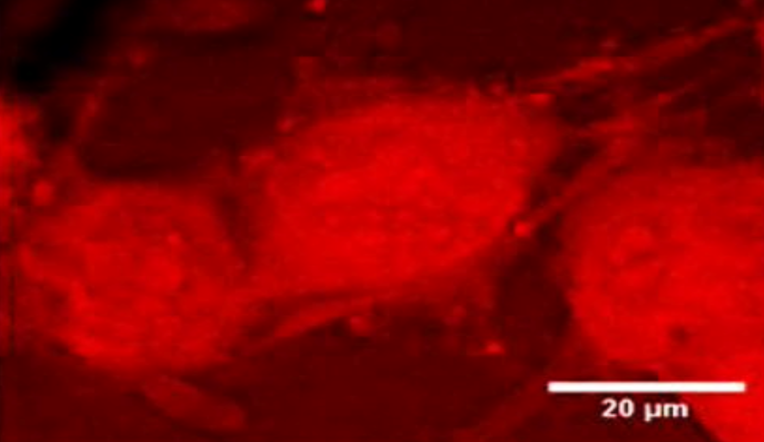
In vitro imaging capabilities of MANTIS. NG108 cells observed with CARS (A) at 2830 cm−1
Wilson R. Adams Sci Rep 11, 8067 (2021).
https://doi.org/10.1038/s41598-021-86774-2
Stimulated Raman scattering (SRS)
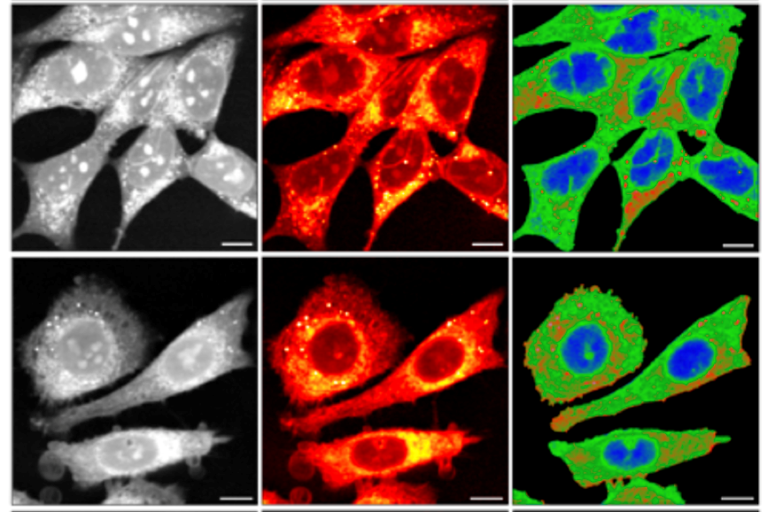
Characterization of prostate cell models using SRS microscopy
Anal. Chem. 2022, 94, 8899−8908, /doi/pdf/10.1021/acs.analchem.2c00236
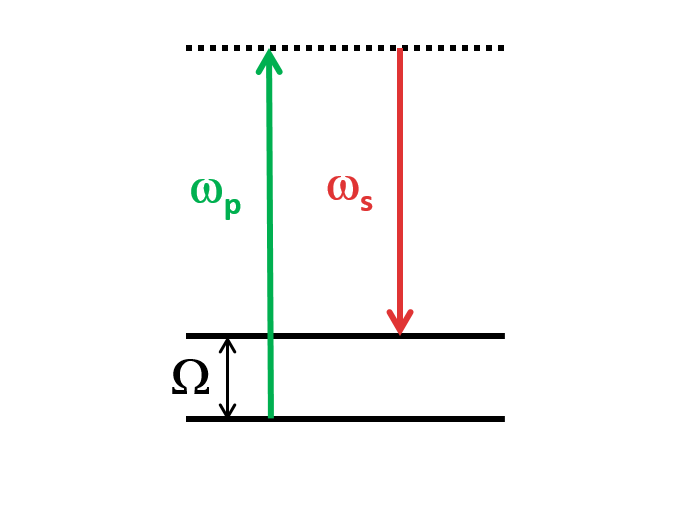
SRS is a nonlinear optical process that occurs when using two laser beams, pump and stokes. The incoming pump light induces intense molecular vibrations and that generate frequency-shifted radiation. SRS can take place when some Stokes photons have previously been generated by spontaneous Raman scattering or when deliberately injecting Stokes photons (“signal light”) together with the pump. That corresponds to the stokes being enhanced (SRG) while reducing the intensity of the exploited pump (SRL). It offers a low NRB (non resonant background), making it ideal for label-free imaging of biological samples. SRS is also fast and non-destructive, allowing for real-time observation of dynamic processes in living systems
Second-harmonic generation (SHG)
SHG, also called frequency doubling, is a nonlinear optical process in which two photons with the same frequency interact with a nonlinear material. These photons are “combined” and generate a new photon with twice the energy of the initial photons (equivalently, twice the frequency and half the wavelength). This process conserves the coherence of the excitation. This process can occur in certain materials that exhibit a non-centrosymmetric crystal structure, such as quartz or potassium dihydrogen phosphate (KDP). SHG has recently emerged as a biophysical tool for conformational sensing of target biomolecules upon binding to ligands such as small molecules, fragments, proteins, peptides and oligonucleotides.<br>
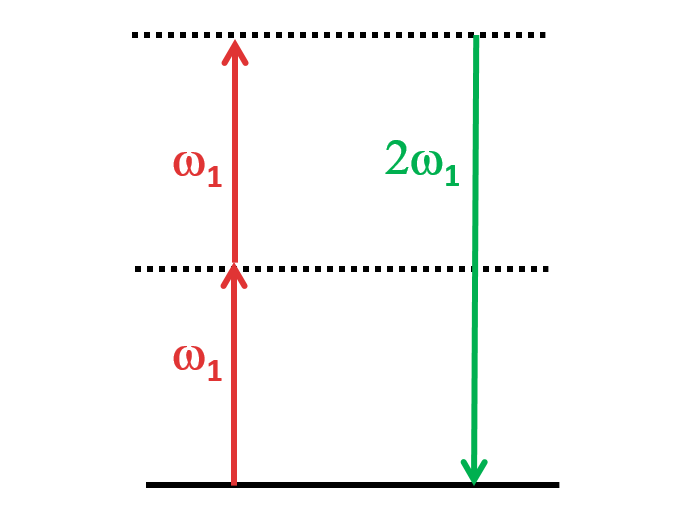
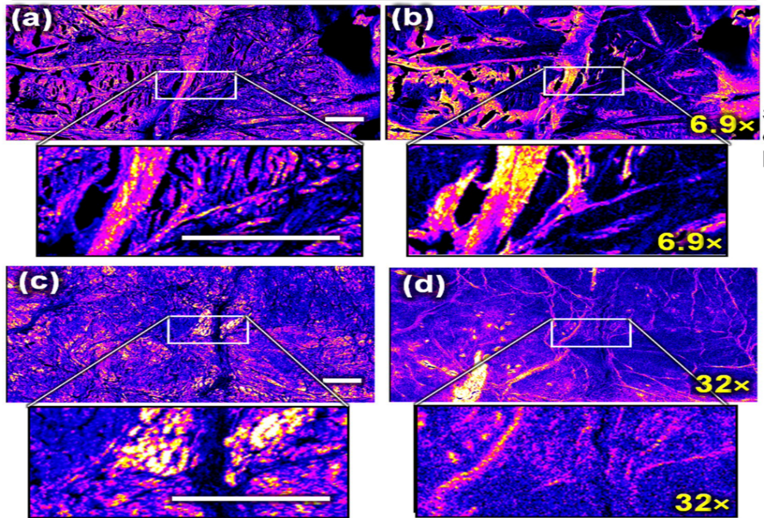
Forward SHG (left, (a,c)) and backward SHG (right, (b,d)) from two menisci samples from equine fetuses
Pinsard, M. al. Sci Rep 9, 18448 (2019). https://doi.org/10.1038/s41598-019-54942-0
Third-harmonic generation (THG)
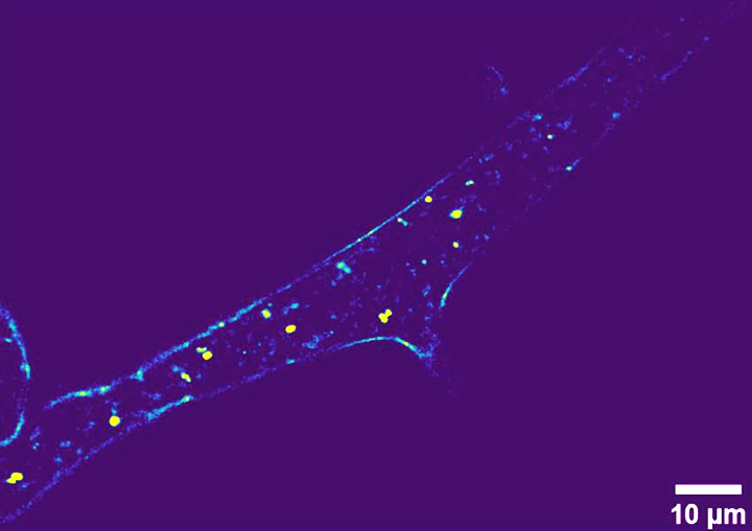
Label-free imaging of Phycomyces blakesleeanus hyphae from the exponential phase in SLM: one THG slice;
Pajić, T. et al. Sci Rep 12, 18760 (2022). https://doi.org/10.1038/s41598-022-23502-4
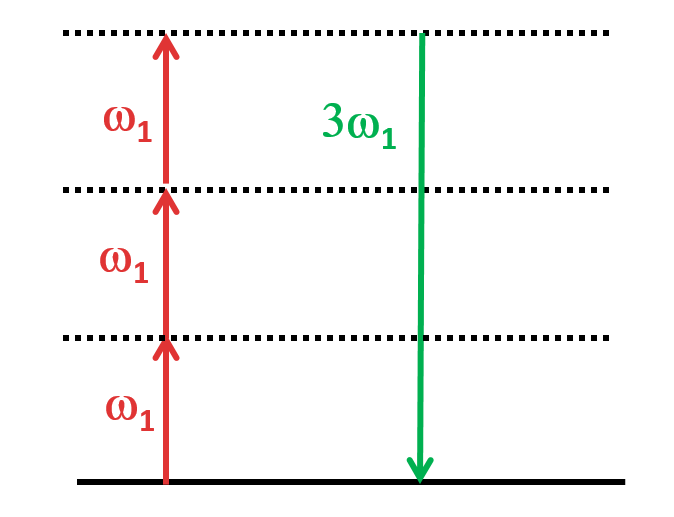
THG is a nonlinear optical process that involves the generation of a third harmonic frequency (wavelength) from an incident beam of light. In THG, three photons of the fundamental frequency interact with a nonlinear medium, such as a crystal or a gas, and are combined to produce a photon with triple the frequency. This process occurs in a narrow region near the surface of the material, where the intensity of the incident light is highest and depends on various factors such as material characteristics and phase matching condition. THG has various applications in fields such as microscopy, spectroscopy, and telecommunications, where it is used to generate ultraviolet or blue light.
Two-Photon Excited Fluorescence (TPEF)
TPEF is a fluorescence imaging technique that allows for the imaging of living tissue. Unlike traditional fluorescence microscopy, in which the excitation wavelength is shorter than the emission wavelength, TPEF uses two photons of lower energy to excite a fluorophore, instead of a single photon of higher energy used in traditional fluorescence microscopy. This technique exploits longer-wavelength excitation light which can penetrate deeper into tissue, allowing for imaging up to about one millimeter in thickness with high spatial resolution. TPEF also has less photobleaching compared to traditional fluorescence microscopy, making it a useful tool in the fields of research that require high-resolution imaging of living tissues.
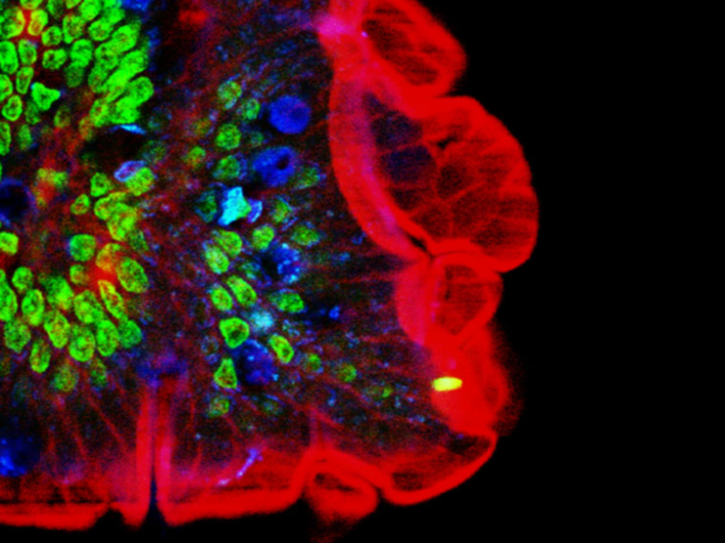
2PE multiple fluorescence image from a 16 μm cryostat section of mouse intestine stained with a combination of fluorescent stains.
Diaspro, A. et al. BioMed Eng OnLine 5, 36 (2006). https://doi.org/10.1186/1475-925X-5-36
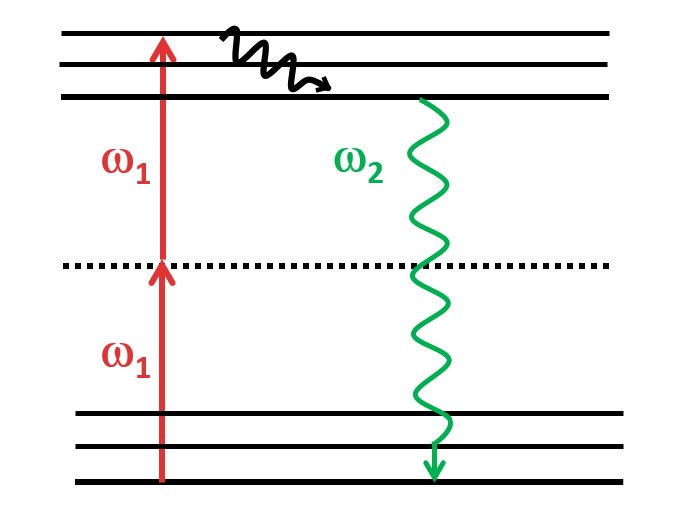
Stimulated-Emission Depletion (STED)
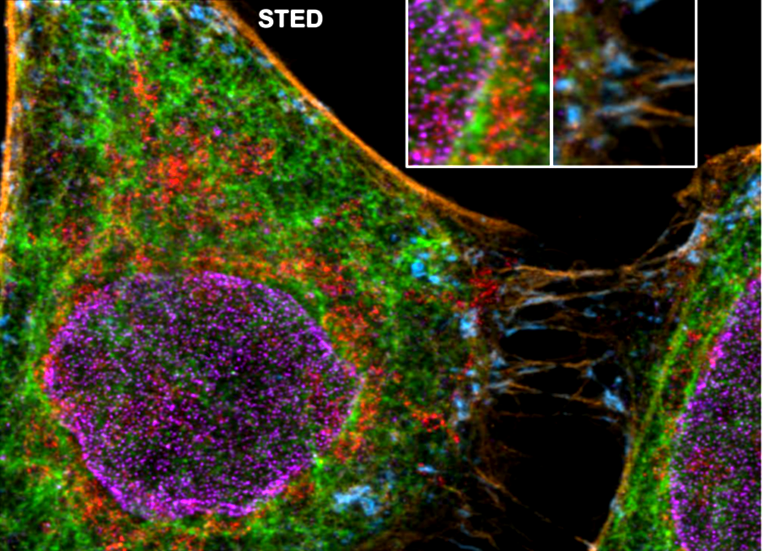
Five color FLIM-STED phasor separated image. Insets show two areas doubled in size.
Gonzalez Pisfil, et al. Sci Rep 12, 14027 (2022). https://doi.org/10.1038/s41598-022-17825-5
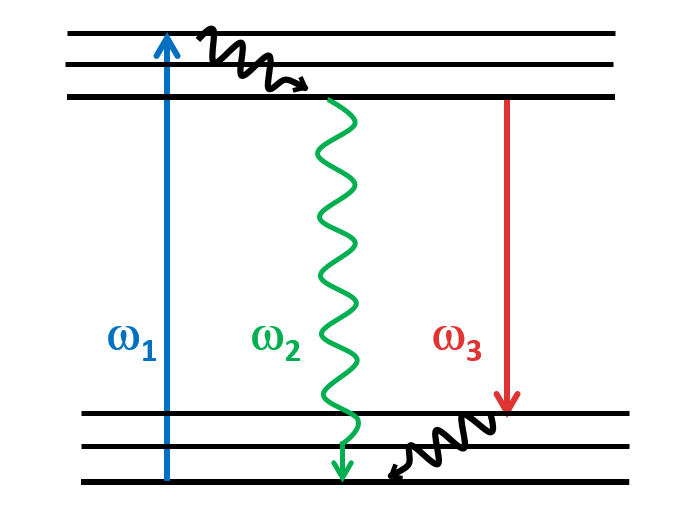
STED is a type of super-resolution microscopy technique that allows to image structures at a resolution much higher than what is possible with conventional light microscopy, beating the diffraction limit. STED is a deterministic functional technique that exploits the non-linear response of fluorophores commonly used to label biological samples. This microscopy technique involves two laser beams: one to excite fluorescent molecules in a sample and a second laser to deplete fluorescence in the outer regions of the excitation spot. By using a second laser to quench fluorescence, the STED microscope can achieve a much higher resolution than conventional fluorescence microscopy. This technique is particularly useful for imaging small structures within cells or other biological samples, as it can provide a much clearer image of the spatial distribution of these structures.